Gut-Lung Axis in Cats: Case Archives (2020-2025) for Further Evidence of Proof in Cats with Several Different Respiratory Patterns Treated with Natural Remedies
K. Ural*, H. Erdoğan, S. Erdoğan, S. Paşa and M. Gültek
Kerem URAL1* (corresponding author), uralkerem@gmail.com, orcid.org/0000-0003-1867-7143;
Hasan ERDOĞAN1, hasan.erdogan@adu.edu.tr, orcid.org/0000-0001-5141-5108; Songül ERDOĞAN1, songultp.09@gmail.com,
orcid.org/0000-0002-7833-5519; Serdar PAŞA1, spasa@adu.edu.tr, orcid.org/0000-0003-4957-9263; Mehmet GÜLTEKİN1,
gultekinmehmet@gmail.com, orcid.org/0000-0002-5197-2403
1 Department of Internal Medicine, Faculty of Veterinary Medicine, Aydın Adnan Menderes University, Aydın, Türkiye
![]() https://doi.org/10.46419/cvj.57.2.8
https://doi.org/10.46419/cvj.57.2.8
Abstract
The gut-lung axis is a crucial bidirectional interaction between intestinal and respiratory microbiota, significantly influencing immune homeostasis. Dysbiosis in these microbial communities has been implicated in various respiratory diseases, including feline asthma. While the gut-lung axis has been extensively studied in humans, its role in feline respiratory pathology remains underexplored. This study aimed to investigate the gut-lung axis in cats by retrospectively analysing cases of feline respiratory disease with the classification of different patterns, assessing the efficacy of probiotic and nutraceutical interventions, and evaluating their impact on clinical outcomes. Case records of 117 cats diagnosed with respiratory distress from 2020 to 2025 were reviewed retrospectively. Respiratory patterns were classified into five groups: inspiratory, restrictive, obstructive, paradoxical, and panting. Diagnostic evaluation included fractional exhaled nitric oxide (FeNO) measurement, thoracic ultrasonography, radiography, and bioresonance analysis. Treatment regimens were individualised based on respiratory pattern classification, incorporating targeted probiotics and nutraceuticals. Statistical analyses, including logistic regression and non-parametric tests, were conducted to assess treatment efficacy. Treatment success varied across respiratory patterns, with the highest response observed in the paradoxical (80%) and obstructive (76.47%) groups, whereas restrictive respiratory patterns exhibited the lowest response rate (62.79%). The presence of multiple B-lines on lung ultrasound, indicative of pulmonary pathology, was significantly associated with restrictive and obstructive breathing patterns (P=0.001). Post-treatment FeNO reduction correlated with clinical improvement, supporting the role of gut microbiota modulation in respiratory disease management. This study provides novel evidence supporting the gut-lung axis in feline respiratory diseases. Tailored probiotic and nutraceutical interventions demonstrated potential therapeutic benefits, particularly in obstructive and paradoxical respiratory distress cases. Future studies should explore microbiome profiling and mechanistic pathways to further elucidate the interplay between gut and lung health in veterinary medicine.
Key words: Gut-lung axis; feline respiratory disease; probiotics; nutraceuticals; microbiota, FeNO; dysbiosis
Introduction
Both the intestinal and respiratory routes of the organism are colonised by trillions of microorganisms, collectively referred to as the microbiota. The microbiota plays a key role in the maintenance of host health and immune response (Gensollen et al., 2016; Zheng et al., 2020). The microbiota of both intestinal and respiratory routes is generally known as complex and dynamic groups that interact with the host’s immune response and other relevant host conditions (Fitzgerald and Kagan, 2020). Research has examined altered microbial compositions, denoting that dysbiosis can make an organism prone to different diseases, such as asthma in humans (Logan et al., 2016; Sharma et al., 2019) and cats (Ural et al., 2021). Dysbiotic alteration in the respiratory and intestinal routes may cause chronic inflammation and could be linked to asthma development. Guinea pigs are commonly used animals to study the possible pathophysiological mechanisms of asthma occurrence (Rosenberg and Druey, 2018).
Taking into account ‘gut-lung axis’ (Budden et al., 2017; Dang and Marsland, 2019; Mach et al., 2021; Vientós-Plotts et al., 2023), further warranted studies are necessary for fulfilling gaps in literature. This aroused our interest several years ago and prompted us to perform this study. At the time of starting this research, the hypothesis was that respiratory disorders should be treated via the gastrointestinal route, taking the gut-lung axis into account. The present study was aimed at investigating the i) gut-lung axis, ii) efficacy of specific probiotic and nutraceutical preference of choice for treating respiratory disease, and iii) clinical reflections, disease progression and treatment outcome of cats with several different respiratory patterns.
Material and Methods
Initial triage
At referral, a group of our assistants involving PhD and MSc students, with a DVM graduate, performed field triage as follows:
Subclassification of cats into three groups: 1) cases that would likely be fatal despite therapeutical intervention, encoded with black (n=33); 2) cats that would likely survive if care was given or not, encoded with green (n=58); and 3) other relevant cats that would likely benefit from simple interventions, encoded with red (n=26) (Wingfield and Palmer, 2009).
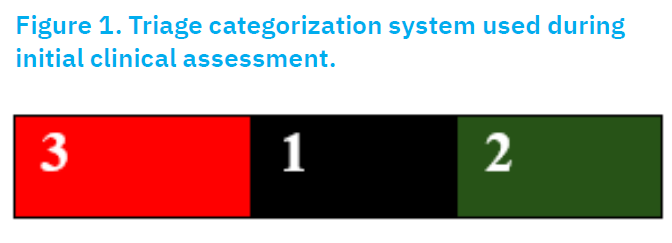
Figure 1. Figure 1. Triage categorisation system used during initial clinical assessment. Cats were classified into three urgency categories based on clinical prognosis at referral: Red (3) = immediate intervention required; Black (1) = fatal prognosis despite treatment; Green (2) = mild cases likely to survive even without advanced care
This classification helped guide diagnostic and treatment priorities in the study.
Respiratory pattern subclassification
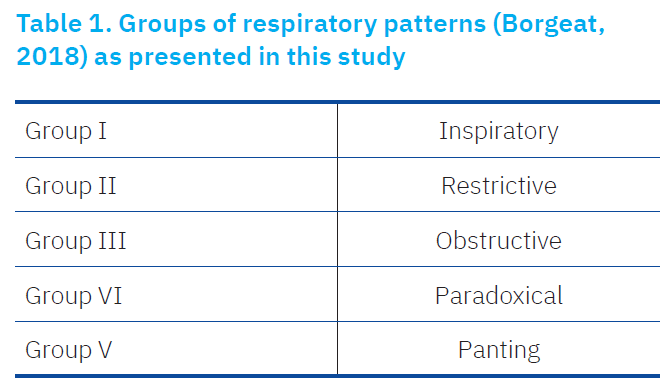
Observation of respiratory pattern is a useful way of narrowing the list of possible differential diagnoses in cats with respiratory distress (Table 1). based on evidence of literature (Borgeat, 2018) respiratory patterns were described at this study.
Respiratory pattern subclassification
In an attempt to observe respiratory patterns, determined as a beneficial route for narrowing differential/probable diagnoses in cats with respiratory distress (Borgeat, 2018), Table 1 shows the patterns used in this study. For this purpose, group were classified according to respiratory patterns evolved.
Demographic approach
The present study is a retrospective collection of available laboratory records and ultrasonograph imaging files at the unofficial Intestinal Permeability Measurement Centre (İPÖM), Faculty of Veterinary, Department of Internal Medicine Isıklı, Aydın, Türkiye. The algorithm developed by İPÖM is shown in Figure 2. The case records for feline referral with respiratory disease were searched from 2014 to late February 2025 for cats subjected to at least one of the following procedures: i) fractional exhaled nitric oxide (FeNO) through a Sunvou Nano Coulomb Breath Analyzer CA2122 (Huang et al., 2021; Lei et al., 2021); ii) abnormal visualisation of thorax ultrasonography, as defined previously (Yang et al., 1992; Reichle and Wisner, 2000; Lisciandro et al., 2008, 2014; Larson, 2009; Lisciandro, 2011; Boysen and Lisciandro, 2015; Dietrich et al., 2015; Chung-Hui et al., 2020); iii) radiographically detected lung parenchymal and pleural space alterations (Lisciandro et al., 2014; Chung-Hui et al., 2020), or iv) bioresonance scanning by Quantum Pet Analysis. For every available record, ultrasonographic imaging data were deemed available for presence/absence for: i) pleural effusion, ii) thick/irregular pleura, iii) comet-tail artifact, iv) lung atelectasis, or v) consolidation, etc., as previously defined by Chung-Hui et al. (2020). Specifical diagnosis for pneumonia involved the presence of thick/irregular pleura, consolidation without nodules or masses (Chung-Hui et al., 2020).
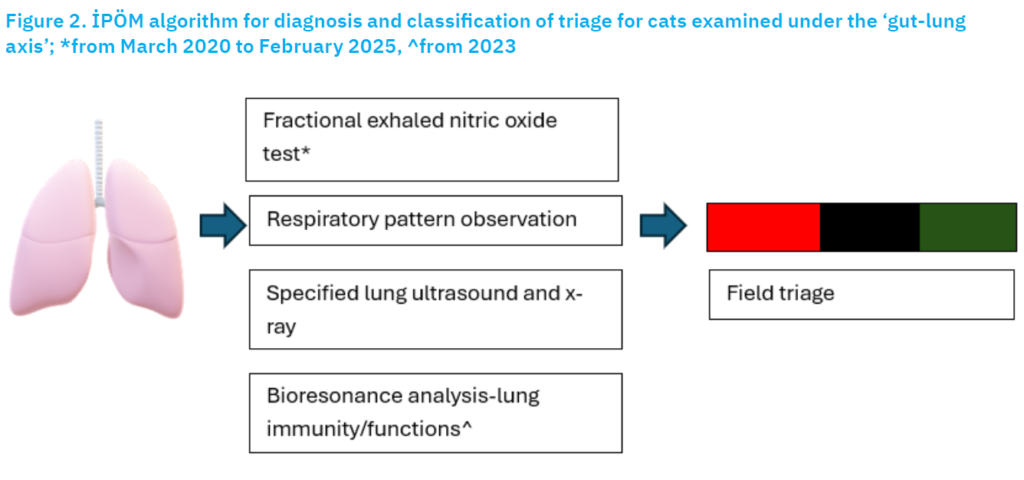
Figure 2. İPÖM algorithm for diagnosis and classification of triage for cats examined under the ‘gut-lung axis’; *from March 2024 to February 2025, ^from 2023
Ethical Statement
This study was reviewed and approved by the Aydin Adnan Menderes University Local Ethics Committee (ADÜ-HADYEK) with document no: 64583101/2023/162. Moreover, written owner consent was available for each cat involved and participated. This research was financially supported by the Aydın Adnan Menderes University Scientific Research Projects Coordination Unit (ADU-BAP) under project number VTF-24006.
Statistical analyses
All statistical analyses were completed using SPSS 29.0 (IBM, USA). Descriptive statistics were evaluated as mean ± standard deviation (SD) and frequencies for categorical variables. The normality of distribution of variables was assessed using the Shapiro-Wilk test. Non-normally distributed continuous and ordinal variables were analysed using the Mann-Whitney U test or Kruskal-Wallis test, as appropriate. Binomial logistic regression analysis was used to evaluate the relationship between respiratory distress and treatment outcomes. Odds ratios (OR) with 95% confidence intervals (CI) were determined for each group. Statistical significance was determined as P<0.05 for all analyses. Logistic regression results were visualised with Forest Plot graphics manually created in Microsoft Excel according to OR and CI values obtained from SPSS outputs.
Treatment modalities
Based on classification of respiratory distress into groups, five different treatment modalities appeared (appraised from the authors’ experience and established cumulative evidence of literature; Ural, 2020; Ural et al., 2020). Both probiotics and nutraceuticals were preferred based on the available literature (Raftis et al., 2018; Aimbire et al., 2019; Groeger et al., 2020; Ural, 2020; Ural, 2022; Zhang et al., 2023; Bezemer et al., 2024; Yoon et al., 2024).

Each probiotic and nutraceutical choices were deemed available based on authors practice and relevant textbooks (Ural, 2020, 2022) and literature (Ural, 2024; Ural and Balıkçı, 2024). Thus, each group received tailored ‘treat to target’ natural substances. Treatment procedures were lasted in 7 to 19 days depending on triage code, laboratory work and evidence of clinical recovery.

Results
Group classification based on both the İPÖM algorithm and relevant literature (Borgeat, 2018) is outlined in Table 3. At the beginning point of study, the authors did not set the categorisation of this study, whereas retrospective interpretation was deemed available. Initial triage changed following treatment intervention in to: black code (n=2), red code (n=69) and gren code (n=46).
As shown above in Table 4, the groups based on the algorithm and relevant literature (Borgeat, 2018) with different respiratory patterns gave lung ultrasounds with varying results. Single B-lines, likely physiological, vs. several B-lines, in accordance with possible lung pathology, were comparatively analysed and an interpretation was deemed available prior to and after treatment. Although no statistical significance was detected, the number of cats with single B lines vs. several B lines was changed in relation with the tailored treatment interventions per group (Table 4).
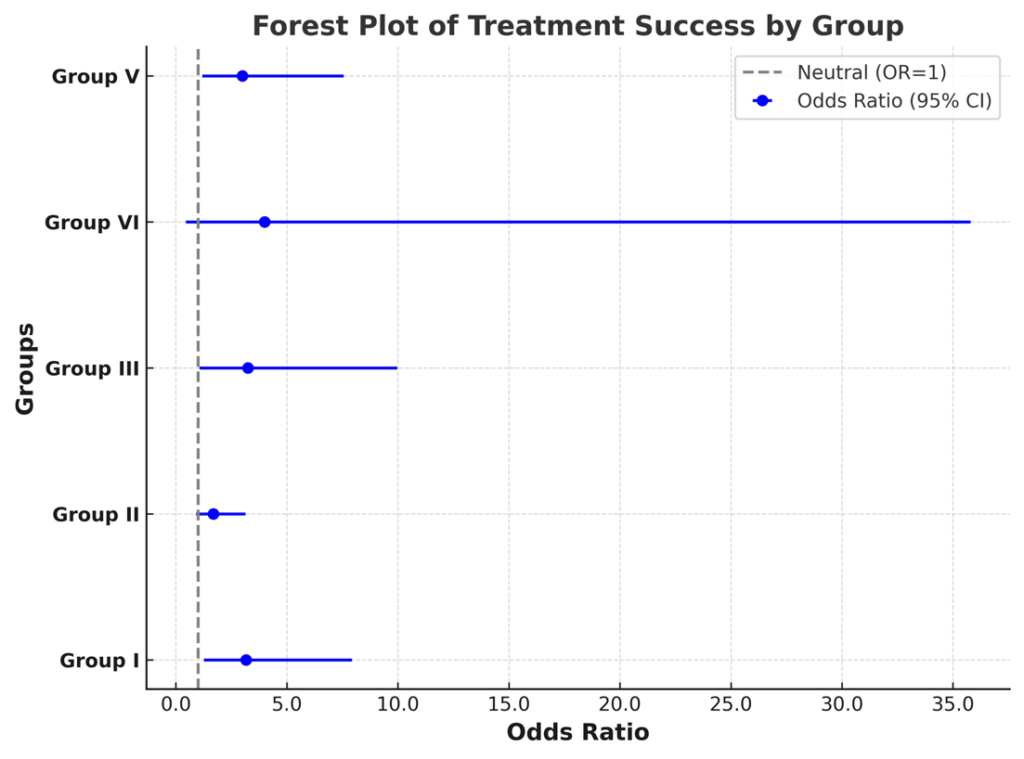
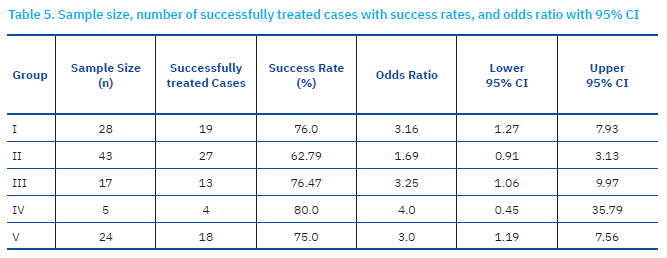
Different groups had different sample sizes, as this was a natural study not an experimental one, based on the available retrospective collection of data. Therefore, group sample size could not be set. Success rates were highest in group IV (80%) and lowest in group II (62.9%). Individual rates for successful treatment were defined as 19/28, 27/43, 13/17, 4/5 and 18/24 from group I to V, respectively (Table 5., Fig 3.).
Figure 3. The Forest Plot illustrates the treatment outcomes across groups
Patients with inspiratory and obstructive breathing patterns demonstrated higher treatment success rates, whereas those with restrictive breathing patterns had the lowest response to treatment. Notably, paradoxical breathing patients exhibited the most favourable response, supporting a distinct pathophysiological mechanism that may contribute to improved therapeutic outcomes. The forest plot (Figure 3) shows an odds ratio of 3.16, 1.69, 3.25, 4.0 and 3.0 for groups I to V, respectively (Table 5). Representative cases revealing the clinical characteristics and therapeutic responses in cats with inspiratory and obstructive respiratory patterns are shown in Figures 4, 5, and 6, respectively.
Figure 4. A cat in group I with inspiratory distress pattern

Figure 5. This cat belonged to group III with obstructive respiratory distress pattern. Feline asthma was diagnosed and responded to tailored treatment protocols as shown in Figure 2

Figure 6. Case in group III with Aelurostrongylus abstrusus infection with obstructive respiratory distress (lower airway disease) that responded to probiotic and nutraceutical treatment. A) during consultation room with elevated respiratory rate, B) after 3 days of treatment with natural compounds targeted ‘gut-lung axis’.
Definition criteria for recovery
As reported above, each group was under interpretation for clinical achievement of related clinical signs and repetitive ultrasonography (also x-ray, fractional exhaled nitric oxide test, bioresonance analytes).
Discussion
The present study, to the authors’ knowledge, is the first in veterinary literature to analyse pulmonary function by use of breath FeNO measurement regarding the ‘gut-lung axis’ in cats. All 117 cats included in the study exhibited altered respiratory functions, and were first subjected to detect further evidence of type 2 inflammation and/or asthma by use of Sunvou-CA2122 Analyzer (NOS), [Nano Coulomb Breath Analyzer CA2122, China], which is capable of measuring the fractional NO concentration in expired breath (FeNO), [guidelines for NO measurement established through the American Thoracic Society] and also based on literature (Huang et al., 2021; Lei et al., 2021). By collecting the breath material into special commercially available bags, measurement of FeNO is non-invasive, simple, and safe. This device further allowed us to determine breath FeNO ≥ 10 ppb (Huang et al., 2021). In total, 56 of 117 cats showed FeNO ≥ 40 ppb, which enabled categorisation based on the presence of type 2 airway inflammation and/or asthma.
The data collected raised a question as to the efficacy of gut microbiota on lung immunity, denoted ‘gut–lung axis’, while the underlying routes and pathophysiology are still being investigated (Marsland et al., 2015). Immune homeostasis, as verified by microbiome and its related components and metabolites (Rooks and Garrett, 2016), might be shifted by antibiotic use, diet and stress, consequently diminishing the abundance of beneficial bacterial species, substituted by the outgrowth of pathogenic species (Hakanson and Molin, 2011). This disturbance of the microbial community, denoted as dysbiosis, rattle tissue and immune homeostasis, is linked to a large spectrum of inflammatory disorders outside the gastrointestinal tract (Shreiner et al., 2015). Perturbation of intestinal–pulmonary cross-talk has been associated with elevated vulnerability to airway infection and disorders, also involving allergy (Keely et al., 2012). The significance of the gut–lung axis (Alic Ural and Ural, 2023) is better understood among cases with chronic gastrointestinal disorders (such as irritable bowel syndrome and inflammatory bowel disease), including humans exhibiting an elevated prevalence of pulmonary disorders (Yazar et al., 2001; Keely et al., 2012; Wang et al., 2013). In the present study, due to financial restraints for performing gut microbiota and lung microbiota analytes, we could not directly conclude gut-lung axis reflection. However, treatment modalities were given in the rectal or oral route, in which modification of the intestinal route was the primary purpose for treating respiratory distress patterns (Alic Ural and Ural, 2023) observed in several cats reported here. Taking into account the forest plot (figure 3), cats with inspiratory (group I) and obstructive (group III) respiratory distress patterns exhibited higher treatment success rates (76% vs 76.47%, respectively) to those of with restrictive respiratory distress patterns which demonstrated the poorest response (62.79%) to treatment. Only five cats were involved with paradoxical respiratory distress pattern exhibiting the most favourable response (80%), supporting a distinct pathophysiological mechanism that may contribute to improved therapeutic outcomes. However, that group (IV) included a very small sample size, which could interfere with success rates.
In the present study, the analysis of B-line distribution and treatment success rates across different respiratory distress patterns revealed distinct trends. The presence of single B-lines (considered physiological) and multiple B-lines (suggesting pulmonary pathology) were assessed among the groups. Statistical analysis using the Chi-square test confirmed significant differences before treatment (p = 0.000028) but not after treatment (p = 0.11444), suggesting a more uniform B-line distribution post-treatment. Patients with restrictive and obstructive breathing patterns exhibited a higher prevalence of multiple B-lines, reinforcing the association between these conditions and pulmonary involvement. In contrast, Group VI (paradoxical breathing) demonstrated the lowest number of B-lines, suggesting a pathophysiology that may not primarily involve pulmonary pathology. For treatment success rates, odds ratios (OR) with 95% confidence intervals (CI) were calculated for each group. The highest treatment success rate was observed in Group IV (80%), with OR = 4.0 (95% CI: 0.45–35.79). However, due to the small sample size, the confidence interval remains wide, indicating uncertainty in the estimated effect size. Group III (obstructive) and Group I (inspiratory) exhibited similar treatment success rates (76.47% and 76.0%, respectively), suggesting comparable therapeutic responsiveness. Conversely, Group II (restrictive) had the lowest improvement rate (62.8%), with OR = 1.69 (95% CI: 0.91–3.13), indicating a relatively lower likelihood of a favourable treatment response. A Kruskal-Wallis test was performed to compare improvement rates across all groups and did not reveal statistically significant differences (p = 0.406), suggesting similar treatment responses among different respiratory distress patterns. Furthermore, pairwise Mann-Whitney U comparisons did not show significant differences between individual group pairs (all p-values > 0.05). This indicates that although there were variations in raw improvement rates, these differences were not statistically significant.
Different treatment modalities were used in this study. This because one model of treatment does not fit for all cats. Each prescription should be different based on disease phenotypes. For instance each probiotic or nutraceutical could not be useful for every case. This treatment modality, as shown in Table 2, is tailored for each individual case. Treat to target purpose allowed us to categorise different respiratory patterns with different nutraceutical and/or probiotic of relevant choices.
In a prior study, the strain Bifidobacterium breve MRx0004 was able to prevent severe asthma model through suppression of both neutrophil and eosinophil lung infiltration in a mouse model (Raftis et al., 2018). Bifidobacterium longum ssp. infantis (B. infantis) has long been known to be able to colonise the infant gut, outcompeting Staphylococcus and Streptococcus, and diminishing inflammation (Frese et al., 2017; Henrick et al., 2021).
Similarly, intranasal Lactobacillus rhamnosus GG also prevented murine asthma (Spacova et al., 2019). During influenza virus infection among mice, the survival rate was elevated by intranasal administration of Lactobacillus, in contrast to the oral route, and receiving live bacteria gave better prevention than receiving dead bacteria. Surprisingly, differences were seen for protection against the influenza virus from several Lactobacillus strains (Youn et al., 2012). Exopolysaccharides from Bacillus subtilis reduced airway inflammation in asthma by diminishing reactive oxygen species and eosinophilic infiltration (Zhang and Yi, 2022).
Conclusion
In the present study, each treatment modality was suited for disease itself. According to different disease phenotypes probiotic or nutraceutical choice were separately preferred.Each treatment modality was unique and based on respiratory pattern itself. it could not be unwise to draw suggestions that ‘bespoke nutraceutical and probiotic treatment’ that has been tailored in association with different respiratory disease patterns could be effective. This novel and natural therapeutical approach is a promising next-generation drug for management of different respiratory patterns detected among cats. Therefore, veterinary practitioners could select of ‘treat to target’ probiotic strains and related nutraceuticals for clinical applications. Moreover practical, true and individually optimised intervention protocols of the selected strains are warranted.
References [… show]
Osovina crijeva-pluća u mačaka: Evidencija slučajeva (2020.-2025.) za daljnje dokazivanje terapije mačaka s različitim respiratornim obrascima liječenih prirodnim lijekovima
Kerem Ural1* (dopisni autor), uralkerem@gmail.com, orcid.org/0000-0003-1867-7143;
Hasan Erdoğan1, hasan.erdogan@adu.edu.tr, orcid.org/0000-0001-5141-5108; Songül Erdoğan1, songultp.09@gmail.com, orcid.org/0000-0002-7833-5519; Serdar Paşa1, spasa@adu.edu.tr, orcid.org/0000-0003-4957-9263; Mehmet Gültekİn1, gultekinmehmet@gmail.com, orcid.org/0000-0002-5197-2403
1 Department of Internal Medicine, Faculty of Veterinary Medicine, Aydın Adnan Menderes University, Aydın, Türkiye
Osovina crijeva-pluća predstavlja najvažniju dvosmjernu interakciju između crijevne i respiratorne mikrobiote, znatno utječući na homeostazu imuniteta. Kod tih mikrobnih zajednica disbioza je prisutna kod različitih respiratornih bolesti, uključujući mačju astmu. Dok je crijevno-plućna osovina široko istraživana u ljudi, njezina uloga u mačjoj respiratornoj patologiji ostaje nedovoljno istražena. Cilj je ove studije istražiti osovina crijeva-pluća u mačaka kroz retrospektivne analize slučajeva mačje respiratorne bolesti, uz kategorizaciju različitih obrazaca, procjenjujući učinkovitost probiotičkih i nutraceutičkih intervencija te ocjenjujući njihov učinak na kliničke rezultate. Evidencija slučajeva 117 mačaka s dijagnozom respiratornih smetnji, retrospektivno se pregledavala od 2020. do 2025. godine. Respiratorni obrasci kategorizirani su u pet skupina: inspiratorna, restriktivna, opstruktivna, paradoksična i dahćuća. Dijagnostička ocjena obuhvaćala je mjerenje djelomično izdahnutog dušičnog oksida (FeNO), torakalnu ultra-sonografiju, radiografiju i analizu biorezonancom. Režimi liječenja bili su individualizirani na temelju kategorizacije respiratornog obrasca, obuhvaćajući ciljane probiotike i nutraceutike. Kako bi se ocijenila učinkovitost liječenja, provođene su statističke analize, uključujući logističku regresiju i neparametarske testove. Uspjeh liječenja varirao je u svim respiratornim obrascima, s najvišim rezultatom kod paradoksičnih (80 %) i opstruktivnih (76,47 %) skupina, dok su restriktivni respiratorni obrasci iskazivali najniže postotke (62,79 %). Prisutnost višestrukih B-linija na ultrazvuku pluća, indikativnih za pulmonalnu patologiju, značajno je povezan s restriktivnim i opstruktivnim obrascima disanja (P=0.001). Redukcija FeNO nakon liječenja korelirana s kliničkim poboljšanjem, podržava ulogu modulacije mikrobiote crijeva kod liječenja respiratornih bolesti. Ova studija daje nove dokaze koji podržavaju koncept os crijeva-pluća u mačjih respiratornih bolesti. Personalizirane probiotičke i nutraceutičke intervencije prikazale su potencijalne terapeutske koristi, posebice kod opstruktivnih i paradoksičkih slučajeva respiratornih smetnji. Buduće bi studije trebale istražiti profiliranje mikrobioma i mehaničke načine za daljnje rasvjetljavanje međuovisnosti zdravlja crijeva i pluća u veterinarskoj medicini.
Ključne riječi: os crijeva-pluća, mačja respiratorna bolest, probiotici, nutraceutici, mikrobiota, FeNO, disbioza