Seroprevalence of Mycobacterium avium subsp. paratuberculosis Infections in Small Ruminants in Europe – A Systematic Review

H. Quintas*, J. Benavides, J. Jacob-Ferreira, P. Afonso and A. C. Coelho
Abstract
Paratuberculosis, also known as Johne’s disease, is a granulomatous enteritis in both domestic and wild ruminants caused by the bacterium Mycobacterium avium subsp. paratuberculosis.
Understanding the prevalence of this disease in small ruminants is essential for disease control and prevention strategies. A systematic review of the literature was conducted using the PubMed, ScienceDirect and Scopus databases to identify all articles reporting Mycobacterium avium subsp. paratuberculosis (MAP) seroprevalence in sheep and goats in Europe, published from January 2006 to December 2023. The initial search for existing publications reporting systematic reviews and primary studies was carried out by searching the available databases.
For the final selection of studies, an initial screen for basic eligibility and a detailed appraisal of quality were performed. After the study selection, the relevant data was extracted. The detailed appraisal generated 21 publications that reported 55 studies, 22 (40.0%) from sheep (12 at the animal-level and 10 at the flock-level) and 28 (50.9%) from goats (17 at the animal-level and 11 at the flock-level), and 5 (9.1%) from mixed small ruminant species at the animal level. In total, 34 (61.8%) were animal-level studies and 21 (38.2%) were flock-level studies. Population and inclusion criteria were highly variable among studies.
Sample sizes ranged from 291 to 15,585 animals.
Most studies reported testing adult animals (over 24 months of age). Commercial ELISA kits were used in most studies. The highest prevalence was obtained in sheep (100%) in Türkiye, and the lowest was found also in sheep (0.7%) in Austria. Overall, the results suggest that MAP antibodies have been frequently detected among small ruminants in some countries and there is a need for standardisation of case definitions to improve the accuracy of prevalence estimates. Further research is needed to understand the risk factors associated with MAP infection in small ruminants and to develop effective control and prevention strategies.Key words: Europe; Mycobacterium avium subsp. paratuberculosis; systematic review; serology
Introduction
Paratuberculosis in small ruminants is caused by Mycobacterium avium subsp. paratuberculosis (MAP), a pathogen dependent on mycobactin, grows slowly and is acid-fast. It is a chronic inflammatory disease affecting the intestinal tract and lymphoid organs. This disease leads to a gradual decline in body weight and is responsible for significant economic losses on a global scale (Idris et al., 2022). Paratuberculosis in small ruminants can cause various clinical signs, including weight loss, decreased milk production, and lethargy. The disease can progress slowly and may not show clinical signs in the early stages (Bauman et al., 2016). In some cases, goats may develop a swollen abdomen due to the thickening of the intestinal wall. Paratuberculosis impacts a variety of wild and domesticated ruminant species, such as sheep, goats, and deer, but also other non-ruminant species such as swine, birds, otters, foxes, camelids, and primates (Miranda et al., 2009, 2011; Matos et al., 2013, 2014, 2015, 2017; Garvey, 2020). Clinical manifestation of paratuberculosis occurs in the final phase of the disease, when sheep and goats can present a variety of clinical signs, including weight loss, decreased milk production, and lethargy. Additional clinical signs in goats include fragile skin, poor coat, submandibular oedema, dehydration, anaemia, and depression (Djønne, 2010). The disease decreases the slaughter value of ruminants, leads to premature culling, reduces milk production and reproductive performance, and raises replacement costs, resulting in significant financial losses for the livestock industry. The potential for MAP-infected animals to contract other illnesses complicates quantifying the actual economic damage (Garcia and Shalloo, 2015; Fanelli et al., 2022). MAP may also be involved in human Crohn’s disease, a chronic inflammatory bowel condition, though this remains under debate (Agrawal et al., 2021; Espeschit et al., 2023).
During the initial stage of infection, MAP is ingested and taken up by macrophages in the intestinal lining. These bacteria can survive and multiply within macrophages, evading the host’s immune system (Whittington et al., 2019).
In a subclinical stage, the infection is managed by a cell-mediated immune response, primarily characterised by a Th1 response. This phase involves the activation of macrophages and the production of key cytokines, such as interferon-gamma (IFN-γ), which are critical in containing the bacteria (Sharma et al., 2024). The immune system relies heavily on cell-mediated immunity, with the generation of T-cells and the activation of macrophages is essential for controlling the infection.
This response is vital for the formation of granulomas, thereby limiting the spread of MAP (Sweeney, 2011). As the disease progresses, there is a notable shift from a cell-mediated (Th1) immune response to a humoral (Th2) immune response. This transition is characterised by the production of antibodies against MAP; however, these antibodies are generally ineffective in controlling the infection (Lee et al., 2023). In this stage, the immune response increasingly favours antibody production, yet this shift is often accompanied by a decline in the overall effectiveness of the immune response. Consequently, this decline allows the bacteria to proliferate and leads to more severe clinical manifestations of the disease (Mallikarjunappa et al., 2021).
Detecting paratuberculosis in animals during its latent period before become infectious is the most crucial factor in eradicating this chronic disease. However, this is difficult with current ante-mortem diagnostic tests. As the treatment is impractical, and despite the availability of a commercial vaccine, paratuberculosis remains a problem in European flocks. Therefore, it is important to assess its spread (Kawaji et al., 2011; Idris et al., 2022).
Although there is no reliable test for detecting MAP infection, several methods have been reported in the literature for diagnosing paratuberculosis and estimating its prevalence, including Ziehl-Neelsen staining bacterial culture, histopathology analysis, PCR of faeces, tissues or blood samples, and diagnostic tools to measure immune response such as agar gel immunodiffusion (AGID), enzyme-linked immunosorbent assay (ELISA), complement fixation tests and Interferon-Gamma Release Assay (IGRA) (Pérez et al., 1996; Garrido et al., 2000; Whitlock et al., 2000; Coelho et al., 2008a, 2008b, 2009, 2010, 2018; Singh et al., 2013; Garg et al., 2015; Buczinski et al., 2019; De Grossi et al., 2020).
ELISA is commonly employed to detect clinically infected animals when the animal may be shedding. However, commercially available ELISA methods have varying sensitivities (Nielsen and Toft, 2009; Blanco et al., 2020). IGRA measures the immune response, specifically the production of interferon-gamma (IFN-γ) by T-cells when exposed to specific MAP antigens. This test can identify the early stages of infection; however, it has several limitations. It is often considered costly and impractical for widespread use, and its reliability can vary. IGRA may not consistently differentiate between infected animals with controlled infections and those with subclinical disease, potentially leading to the culling of healthy animals (Bulum et al., 2024). ELISA tests are the most logical option for epidemiological studies as they are cost-effective, although they will miss a percentage of subclinically infected animals (Swenney, 2011).
Diagnosing paratuberculosis in small ruminants based solely on clinical signs is inadequate, but by combining these signs with epizootiological data, it is possible to suspect the presence of MAP at the animal level (Whittington et al., 2019).
The seroprevalence of MAP infections in small ruminants is a significant concern across Europe. When making decisions or policies regarding an infection, the prevalence among the herd and individual animals is frequently a crucial factor that decisions or policymakers consider when determining the significance of infection and the appropriate measures to be taken (Nielsen and Toft, 2009). Understanding the current seroprevalence of the disease at both the herd and animal levels is necessary to prioritise and guide future research and control programmes (Rita et al., 2011).
Conducting a systematic review of the seroprevalence of paratuberculosis in small ruminants in Europe is of great importance for understanding the extent of the problem and developing effective prevention and control strategies. Authorities can put particular management measures in place to stop the spread of MAP among livestock by determining the prevalence of the disease. This systematic review aimed to gather and critically evaluate the available scientific evidence on the seroprevalence of paratuberculosis in small ruminants in Europe in recent years to provide a comprehensive overview of the current state of the disease and identify research gaps.
Material and Methods
Study design
The present study consisted of a systematic literature review to answer the following research question: What is the seroprevalence Mycobacterium avium subsp. paratuberculosis reported in sheep and goats in Europe in the last 17 years?
This study was conducted based on the methodological recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021).
Article eligibility
Articles published in indexed journals cited in PubMed, ScienceDirect and Scopus were considered eligible if they consisted of research papers, short communications, and cross-sectional studies describing serological features (including species, population, prevalence/occurrence, and country). There were other restrictions regarding the eligibility criteria: only studies published between 1 January 2006 and 31 December 2023 and written in languages of Western Europe (comprising English, French, German, Italian, Portuguese, and Spanish) were included.
Publications included original research papers, short communications and cross-sectional studies that addressed issues within the following criteria: seroprevalence, small ruminants, sheep, goats, and Europe. Publications, including studies reporting seroprevalence, frequency, occurrence, or proportion of paratuberculosis in individuals and/or flocks located in European countries performed by serological methods in blood, were included.
Reviews of the literature, case reports, research notes, editorials, experimental essays, textbook chapters, posters, abstracts, articles with no primary data, dissertations, and unpublished studies and data were excluded.
Information sources and search strategies
The process of identifying articles in indexed journals was developed using PubMed (available at http://www.ncbi.nlm.nih.gov/pubmed), Scopus (http://www.scopus.com), and ScienceDirect (https://www.sciencedirect.com/) databases. Studies conducted on other farm animal species or wild ruminants were excluded.
The combination of search terms in English applied included: {[Europe] AND [Mycobacterium avium subsp. paratuberculosis OR paratuberculosis OR Johne´s disease] AND [small ruminant OR sheep OR ovine OR goat OR caprine] AND [seroprevalence OR prevalence OR occurrence OR geoepidemiology OR ELISA OR Serology OR Serological diagnosis OR seropositivity]}. To prevent missing data, references of retrieved publications were also checked to identify additional papers. The searches were conducted between January 2023 and March 2024.
After a comprehensive systematic search, duplicate records were excluded.
Then, two independent reviewers selected articles based on their titles and abstracts, followed by a complete reading of the text when the title or abstract met the inclusion criteria or could not be rejected with certainty. Any disagreements or divergences were resolved by discussion and consensus. Two researchers extracted the required data and added the information on an electronic spreadsheet, dividing them into five groups: (i) sheep, (ii) goat, (iii) sheep flock, (iv) goat flock, (v) small ruminant flock. The initial screen for basic eligibility was carried out using titles and abstracts of relevant studies found by searching databases and reference lists through the snowballing procedure for reports that match the general inclusion criteria on population, study factor, and outcome. Following the preliminary screening, a thorough quality assessment was carried out. Full-text versions of potentially eligible studies were evaluated for three principal issues: minimisation of selection bias of animals or flocks, an adequate assertion of outcomes, and minimisation of measurement or misclassification bias.
Data extraction
After the final selection of studies, quantitative data was extracted and included the reference (authors and year of publication), data on country and region, study period, population, inclusion criteria, selection, sensitivity, and specificity used (if available) for adjusting prevalence estimation, and results in terms of proportion were extracted. Studies from which no crude numbers on frequency or proportion are included were excluded unless the information provided allowed its calculation.
Results
Selection of Papers
The search of the databases yielded 2122 results, once duplicates had been removed (Figure 1).
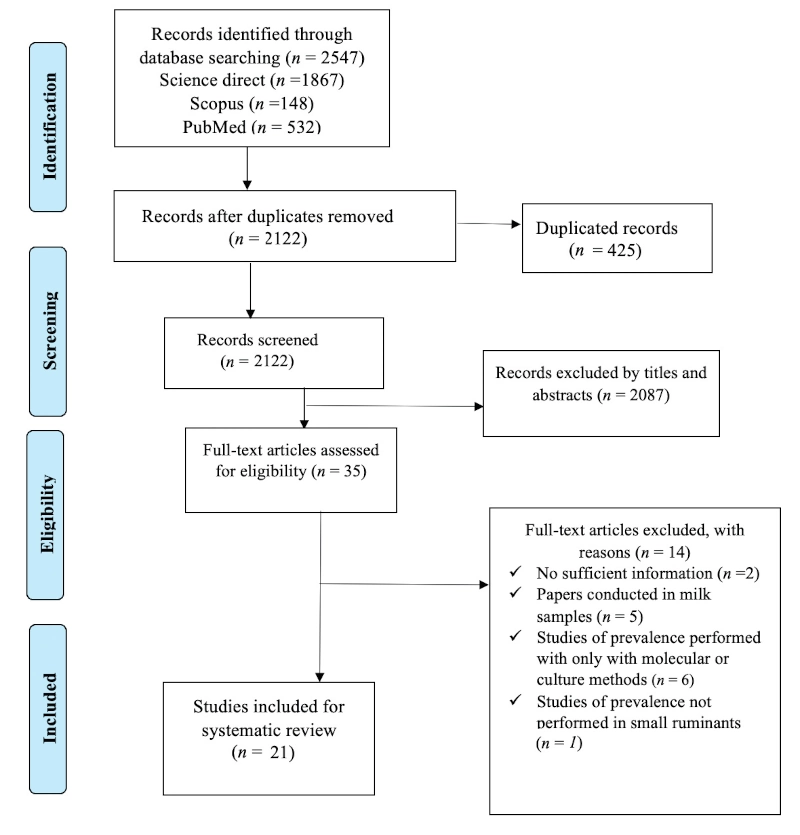
After screening titles and abstracts, 35 records potentially met the inclusion criteria. From the analysis of their full text, 14 articles were excluded, and 21 studies were included in this systematic review (Coelho et al., 2007; Ikonomopoulos et al., 2007; Falconi et al., 2009; Liapi et al., 2011; Rita et al., 2011; Corrias et al., 2012; Stau et al., 2012; Márquez et al., 2013; Buyuk et al. 2014; Mercier et al., 2015; Graham et al., 2016; Celik and Turutoglu, 2017; Galiero et al., 2017; Barrero-Domínguez et al., 2019; Cecchi et al., 2019; De Grossi et al., 2020; Iarussi et al., 2019; Quintas et al., 2021; Jiménez-Martín et al., 2022; Gaffuri et al., 2023; Schrott et al., 2023).
The study results were analysed according to animal species at the animal and flock levels. Articles that did not meet the inclusion criteria and duplicates were excluded.
Qualitative analysis of the prevalence of Mycobacterium avium subsp. paratuberculosis in small ruminants
The final analysis included 21 of 35 eligible publications after quality appraisal. The 21 selected publications included 55 studies: 22 (40.0%) from sheep (12 at the animal-level and 10 at the flock-level), 28 (50.9%) from goats (17 at the animal-level and 11 at the flock-level), and 5 (9.1%) from mixed small ruminant species at the animal level. In total, 34 (61.8%) were animal-level studies, while 21 (38.2%) were flock-level studies (Tables 1, 2, 3, 4 and 5).
The studies were carried out in 10 countries: Austria (n=5; 9.1%), France (n=2; 3.6%), Germany (n=5; 9.1%), Greece (n=5; 9.1%), Italy (n=15; 29.3%), Portugal (n=4; 7.3%), Cyprus (n=4; 7.3%), Scotland (n=1; 1.8%), Spain (n=8; 14.5%), and Türkiye (n=6; 10.9%).
Forty-five studies reported information on the study period, and these studies were carried out between 2003 to 2021. However, they were required to have been published between 2006 and 2023.
The diagnostic tests used in the selected studies were ELISA in 50 studies and the complement fixation test in five studies.
High variability was observed in the commercial ELISA diagnostic tests. Only 36 studies provided information on the sensitivity and specificity used for prevalence adjustment or true prevalence estimation.
The remaining studies provided only apparent prevalence.
All studies reported information on the selection of animals or flocks included for MAP in small ruminant testing in the studies. Random selection of animals or flocks was reported in 54.5% (30/55) of the studies. Six studies were stratified by random sampling. Studies that report convenience sampling were kept in the study to summarise and synthesise their results if animals or flocks had no previous diagnosis of paratuberculosis.
Prevalence in sheep at the animal level
Prevalence studies in sheep at the animal level (n = 12) reported in nine countries are shown in Table 1.
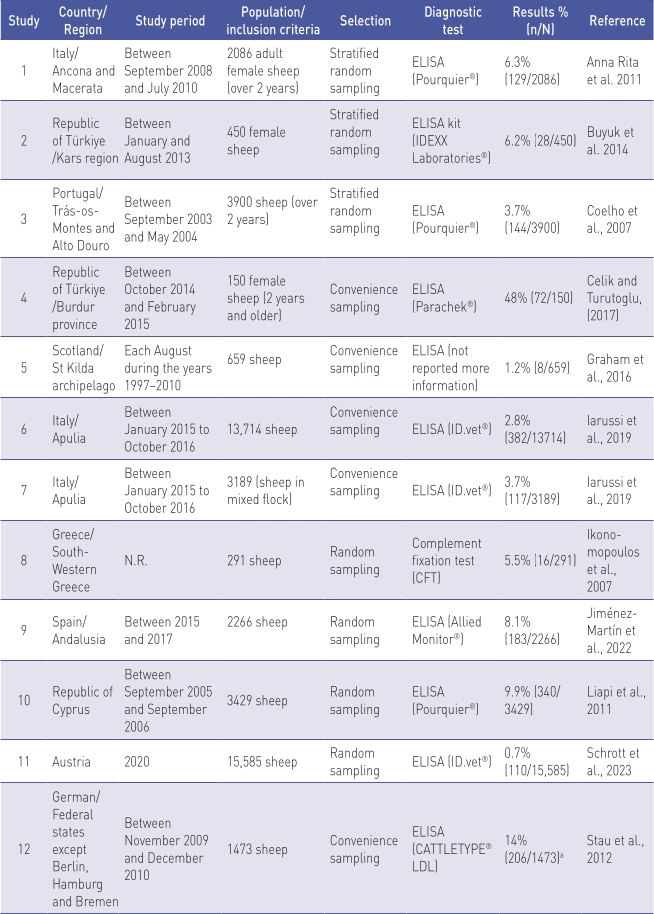
N.R. Not reported.
a Results of proportion were absent from the publication but were calculated using the data on sample size and apparent prevalence.
Of the studies included in the final analysis, three (25%) were from Italy, two (16.7%) from Türkiye, and one each (8.3%) from Austria, Germany, Greece, Portugal, Scotland, Spain, and Cyprus. Information on the study period was provided in 91.7% (11/12) of the studies.
Population and inclusion criteria were highly variable among studies. Sample sizes ranged from 291 (Ikonomopoulos et al., 2007) to 15,585 animals (Schrott et al., 2023).
Most studies tested adult animals (i.e., over 24 months). A commercial ELISA kit was used in 91.7% of the cases (11/12).
The highest prevalence was obtained in the Republic of Türkiye (48.0%) (Celik and Turutoglu, 2017). The lowest level (0.7%) was detected in Austria (Schrott et al., 2023).
Prevalence in goats at the animal level
Prevalence studies in goats at the animal level (n=17) are shown in Table 2.
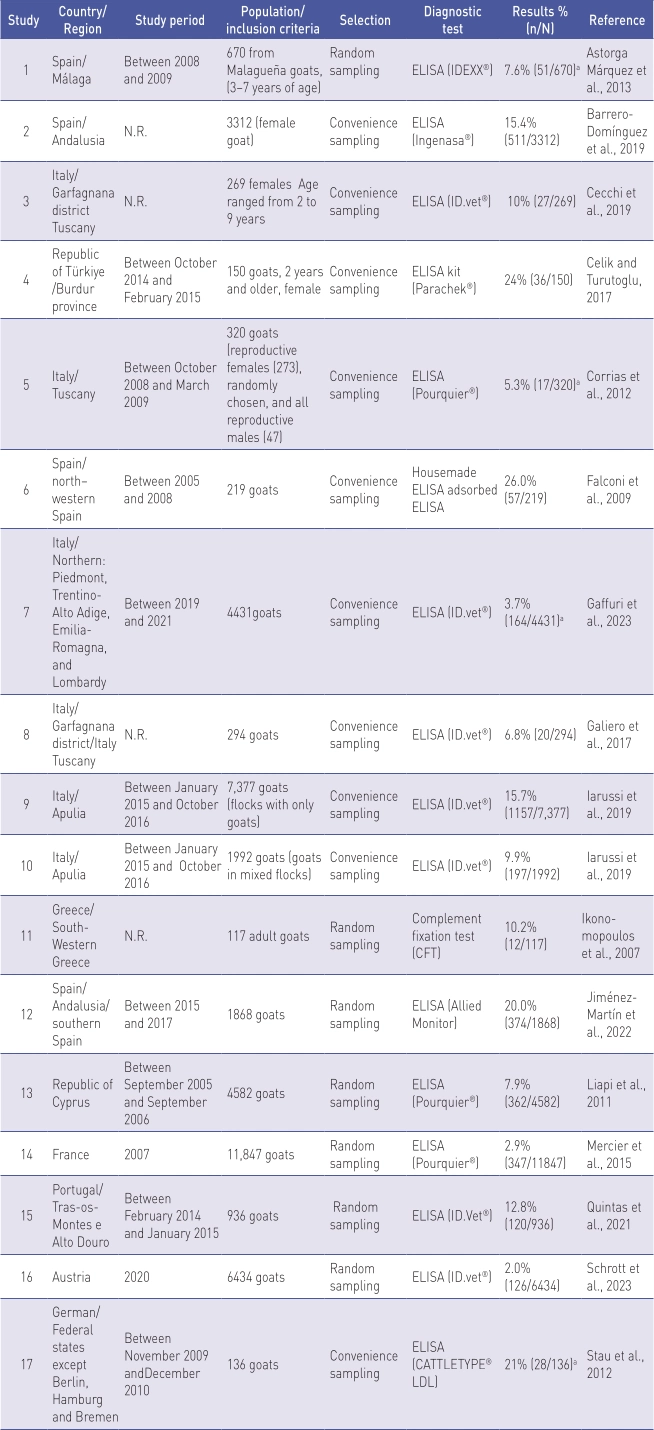
N.R. Not reported.
a Results of proportion were absent from the publication but were calculated using the data on sample size.
The studies included in the final analysis were from Austria (n=1; 5.9%), France (n=1; 5.9%), Germany (n=1; 5.9%), Greece (n=1; 5.9%), Italy (n=6; 35.3%), Portugal (n=1; 5.9%), Cyprus (n=1; 5.9%), Spain (n=4: 23.5%), and Türkiye (n=1; 5.9%).
Most studies did not provide the age of the animals included in the sample.
All studies reported information on the selection of animals tested for MAP. In these studies, random sampling was used in 41.2% (n=7) and convenience sampling was used as the selection procedure in 58.8% (n=10). The ELISA kit used to detect individual cases in the studies was diverse. Twelve (70.6%) studies reported information on the sensitivity and specificity of the test used.
Prevalence studies in goats at the animal level in European countries showed a prevalence that ranged between 2.0% and 26%. The highest paratuberculosis prevalence in goats at the animal level was found in Spain (26.0%) (Falconi et al., 2009). Low prevalence was obtained in Austria (2.0%) (Schrott et al., 2023) and France (2.9%) (Mercier et al., 2010).
Flock level prevalence in sheep
Flock level prevalence studies in sheep (n=10) are shown in Table 3.
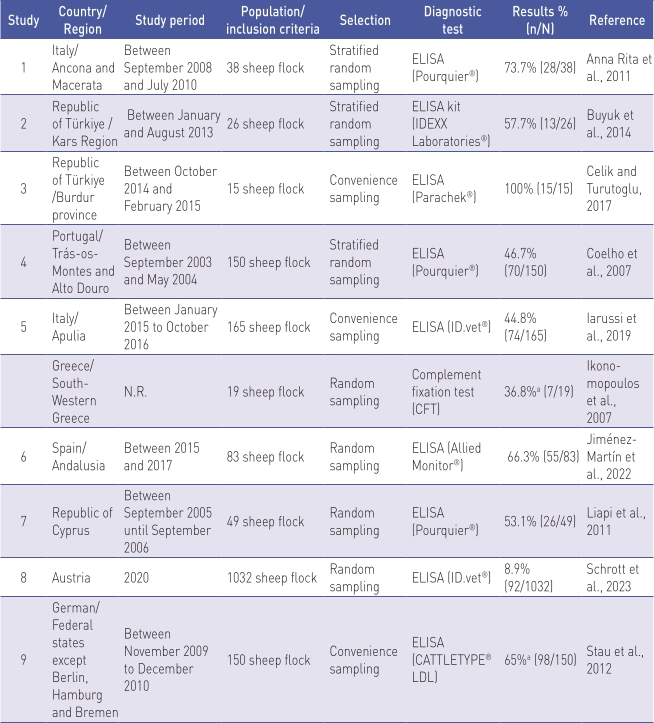
N.R. Not reported.
a Results of proportion were absent from the publication but were calculated using the data on sample size and apparent prevalence.
The studies were carried out from 2003 to 2020, though one study did not report the study period. Population and inclusion criteria differed between the studies. Sample sizes ranged from 19 (Iknomopoulos, 2007) to 1032 (Schrott et al., 2023). In these studies, random sampling was used in 70% (n=7) of studies (three studies used stratified random sampling), and convenience sampling was used as the selection procedure in 30% (n=3). In 70% (n=7) of studies, information on sensitivity and specificity was reported. ELISA tests were used in 90% (n=9) of studies.
Flock level prevalence studies in sheep in European countries revealed a prevalence between 8.9% (Schrott et al., 2023) and 100% (Celik and Turutoglu, 2017).
Flock level prevalence in goats
Table 4 displays the results of flock level prevalence studies conducted in goats (n=11).
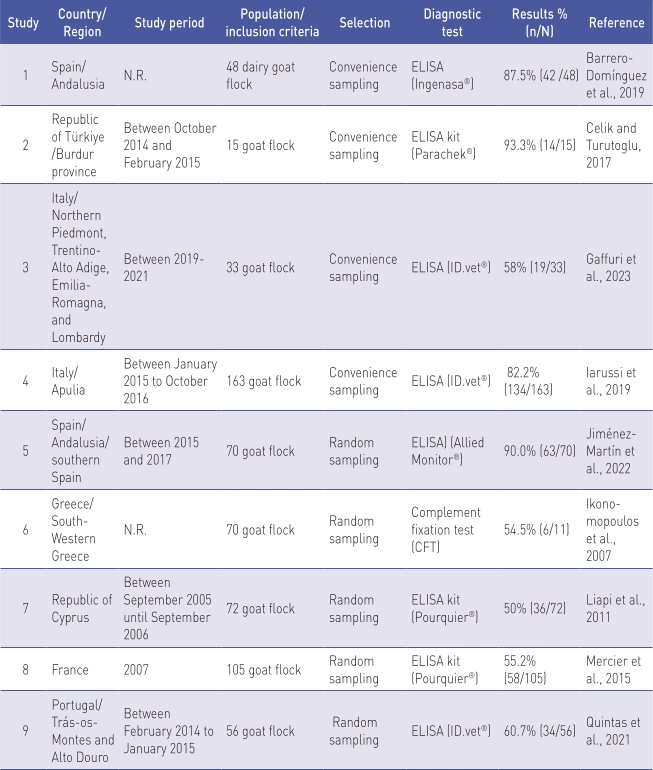
N.R. Not reported.
a Results of proportion were absent from the publication but were calculated using the data on sample size.
The studies were conducted between 2005 to 2021. Population and inclusion criteria differed between the studies. Sample sizes ranged from 15 (Celik and Turutoglu, 2017) to 638 (Schrott et al., 2023) goat flocks. As the selection procedures, random sampling was used in 54.5% (n=6) studies, and convenience sampling was used in 45.5% (n=5). In 63.7% (n=7) studies, information on sensitivity and specificity was reported. ELISA tests were used in 90.9% (n=10) of studies.
Goat flock level prevalence studies in European countries have shown that the prevalence ranges between 1.1% (Schrott et al., 2023) and 93.3% (Celik and Turutoglu, 2017).
Individual level prevalence in small ruminants
Table 5 shows prevalence studies for small ruminants at the animal level (n=5).
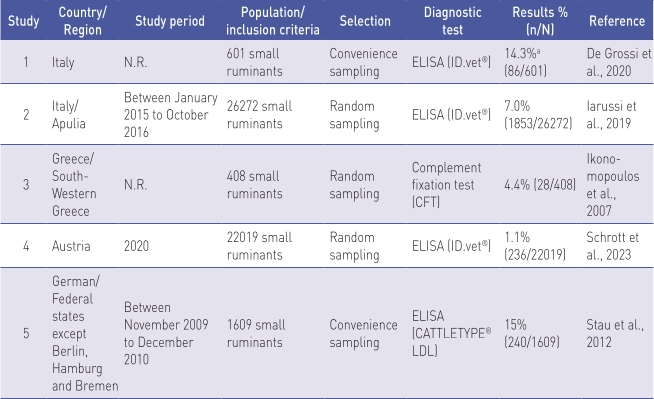
N.R. Not reported
a Results of proportion were absent from the publication but were calculated using the data on sample size and apparent prevalence.
Of the studies included in the final analy- sis, one each was from Austria, Germany, and Greece, and two were from Italy.
The study population and inclusion criteria were highly variable. Sample siz- es ranged from 408 (Ikonomopoulos et al., 2007) to 26,272 (Iarussi et al., 2019) small ruminants.
A commercial ELISA kit was used in four of five studies. Random sampling was used as the selection procedure in three of five studies.
The lowest level (1.1%) was detected in Austria (Schrott et al., 2023) and the high- est prevalence (15.0%) was obtained in Germany (Stau et al., 2012).
Discussion
The seroprevalence of MAP in sheep and goats across Europe has been studied extensively due to its significant impact on animal health and agricultural productivity. This systematic review summarises individual and flock MAP seroprevalence throughout European countries and describes the diagnostic tests employed in recent decades. Detailed knowledge of the seroepidemiological status of paratuberculosis in sheep and goats is essential for assessing effective prevention measures against this disease in domestic animals (Whittington et al., 2019).
A systematic methodology was used to review studies on the prevalence of MAP in European countries (Coelho et al., 2007; Ikonomopoulos et al., 2007; Falconi et al., 2009; Liapi et al., 2011; Rita et al., 2011; Corrias et al., 2012; Stau et al., 2012; Márquez et al., 2013; Buyuk et al. 2014; Mercier et al., 2015; Graham et al., 2016; Celik and Turutoglu, 2017; Galiero et al., 2017; Barrero-Domínguez et al., 2019; Cecchi et al., 2019; De Grossi et al., 2020; Iarussi et al., 2019; Quintas et al., 2021; Jiménez-Martín et al., 2022; Gaffuri et al., 2023; Schrott et al., 2023). We aimed to gather all possible evidence on the frequency or seroprevalence of paratuberculosis in Europe. We assumed that there are more similarities in production conditions, breeds, historical processes, and climates among European countries compared to countries in other regions of the world, such as America, Australia, or Asia. The number of publications on paratuberculosis in Europe was greater than expected, indicating an upward trend in paratuberculosis research and a growing interest in this disease and its negative effects. However, the number of publications and studies focused on estimating the seroprevalence or frequency of antibodies MAP was relatively low, although it was comparable to that of a previous review on the same topic in Europe (Nielsen and Toft, 2009).
The limited number of articles in this systematic review on paratuberculosis seroprevalence in these species suggests that research in this area is insufficient.
Over the past two decades, paratuberculosis has been classified as a notifiable disease in 65% of the 43 European countries examined. However, fewer than 50% of these countries have consistently implemented passive surveillance, and only 19% have engaged in active surveillance (Fanelli et al., 2022). This indicates that paratuberculosis may not be a priority for animal health in many European nations.
The absence of relevant information for study quality assessment was previously reported in Europe in a review of prevalence in farm animals (Nielsen and Toft, 2009). In the present review, case definitions, as well as variability in the kits of diagnostic tests and lack of random sampling for animal and flock selection, were the main study flaws included in the final analysis.
The present systematic review on the seroprevalence of sheep and goat paratuberculosis over the last 17 years revealed highly variable seroprevalence among studies, ranging from 0.7% found in sheep in Austria (Schrott et al., 2023), up to 100% in sheep in Türkiye (Celik and Turutoglu, 2017).
The variability in the prevalence rate of MAP in small ruminants can be attributed to several factors, including geographic location and various unmeasured factors such as climate, production system regime, mechanisation, and professional assistance (Rangel et al., 2015). Improved diagnostic techniques and increased awareness may have led to better detection rates in some areas. Conversely, the implementation of control programmes in certain countries might have contributed to a reduction in prevalence.
The present review refrained from attempting the calculation of true prevalence due to the variability of tests used, and a lack of information on the sensitivity and specificity in some studies conducted in the populations under study.
However, we were careful to exclude any studies that clearly exhibited selection bias toward clinical animals or animals with a history or diagnosis of paratuberculosis, to maintain the integrity and validity of our review. The diversity of diagnostic kits and other factors not studied in this work, such as the type of control programmes in those countries/regions, vaccination, and production system used, could explain the high heterogeneity of results from ELISA-based studies.
Our systematic review has several limitations. First, there is a chance that not all the publications related to sheep and goat paratuberculosis were included during document retrieval from the selected databases, although three search databases (PubMed, Scopus, Science Direct) were used, partially because of the keyword selection in the publication itself.
The quality of evidence regarding the seroprevalence of paratuberculosis in the region is restricted due to various flaws in the study design. The main weakness of these studies was the absence of a clear definition of cases, variability in selected populations, and inclusion criteria for animals and flocks, which could affect the accuracy of prevalence estimates. Another limitation is the high variability in the commercial ELISA diagnostic tests used, variability or lack of information on sensitivity and specificity of diagnostic tests, and lack of random selection of animals and flocks, which also affect the accuracy of prevalence estimates. It is important to note that the apparent increase or decrease in seroprevalence may also be partly due to methodological differences between studies. This underscores the need for standardised approaches in future epidemiological studies to facilitate more accurate comparisons across time and regions.
Conclusions
This systematic review has provided a comprehensive analysis of MAP seroprevalence in sheep and goats across Europe.
The review highlights significant variability in seroprevalence that can be attributed to several factors, including geographic differences, climate, production systems, diagnostic methods, and the presence or absence of formal control programmes.
Despite the upward trend in research and growing interest in paratuberculosis, our findings underscore a notable insufficiency in the number of high-quality studies focusing on MAP seroprevalence in small ruminants in Europe. The limited number of articles, coupled with common methodological flaws such as inconsistent case definitions, variability in diagnostic tests, and lack of random sampling, suggests a need for more robust and standardised research approaches in this field. While significant progress has been made in understanding the seroprevalence of MAP in European small ruminants, there remains a critical need for more rigorous, standardised, and comprehensive research.
Such efforts will be essential in developing effective prevention and control measures, ultimately enhancing European animal health and agricultural productivity.
References [… show]
Seroprevalencija infekcije s Mycobacterium avium subsp. paratuberculosis u malih preživača u Europi – sistematski pregled
Hélder QUINTAS, DVM, MSc, PhD, Mountain Research Center (CIMO), Bragança Polytechnic University (IPB), Campus de Santa Apolónia, 5300-253 Bragança, Portugal; Júlio BENAVIDES, Instituto de Ganadería de Montaña, CSIC-University of Leon, Grulleros, 24346 León, Spain; João JACOB-FERREIRA, CIMO, IPB, Campus de Santa Apolónia, Bragança, 5300-253, Portugal; Paulo AFONSO, DVM, MSc, PhD, Animal and Veterinary Research Centre (CECAV), University of Trás-os-Montes e Alto Douro (UTAD), Quinta de Prados, 5000-801 Vila Real, Portugal; and CIMO, IPB, Campus de Santa Apolónia, 5300-253 Bragança, Portugal; Ana Cláudia COELHO, DVM, MSc, PhD, Department of Veterinary Sciences, School of Agrarian and Veterinary Sciences (ECAV), CECAV, UTAD, Quinta de Prados, 5000-801 Vila Real, Portugal
Paratuberkuloza, poznata i kao Johneova bolest, je granulomatozni enteritis u domaćih i divljih preživača koju prouzroči bakterija Mycobacterium avium subsp. paratuberculosis. Razumijevanje prevalencije ove bolesti u malih preživača ključno je za strategije kontrole i prevencije bolesti. Sistematski pregled literature je proveden uporabom PubMed, ScienceDirect i Scopus baza podataka za pronalaženje svih članaka u svezi seroprevalencije Mycobacterium avium subsp. paratuberculosis u ovaca i koza u Europi, objavljenih od siječnja 2006. do prosinca 2023.
Početna pretraga postojećih publikacija koje su donosile sistematski pregled i izvijestile o primarnim studijama provedena je pretragom dostupnih baza podataka. Za konačni odabir studija, obavljen je početni probir osnovne podobnosti i detaljna procjena kvalitete. Nakon odabira studija, izvedeni su odgovarajući podatci. Detaljna procjena generirala je 21 publikaciju s izvješćima o 55 studija, 22 (40,0 %) na ovcama (12 na razini životinja i 10 na razini stada) i 28 (50,9 %) na kozama (17 na razini životinja i 11 na razini stada) te 5 (9,1 %) na različitim malim preživačima na razini životinja. Sveukupno, 34 (61,8 %) bile su studije na razini životinja, dok je 21 studija (38,2 %) bila na razini stada. Populacija i kriteriji uključenja vrlo su se razlikovali među studijama. Veličina uzorka kretala se između 291 do 15.585 životinja.
Većina studija izvještavala je o testiranju odraslih životinja (tj. više od 24 mjeseci starosti). Komercijalni ELISA kompleti su rabljeni u većini studija. Najveća prevalencija dobivena je u ovaca (100 %) u Republici Turskoj, a najmanja je otkrivena u ovaca (0,7 %) u Austriji. Općenito, rezultati su ukazali da se MAP antitijela često otkrivaju među malim preživačima u nekim zemljama i da postoji potreba za standardizacijiom definicija slučajeva da bi se poboljšala točnost procjena prevalencije. Potrebno je dodatno istraživanje da bi se razumjeli faktori rizika povezani s MAP infekcijom u malih preživača i za razvoj učinkovitih strategija kontrole i prevencije.Ključne riječi: Europa, Mycobacterium avium subsp. paratuberculosis, sistematski pregled

